For this unit of quantitative chemistry, there are 3 key formulas for mole conversion that you need to remember.
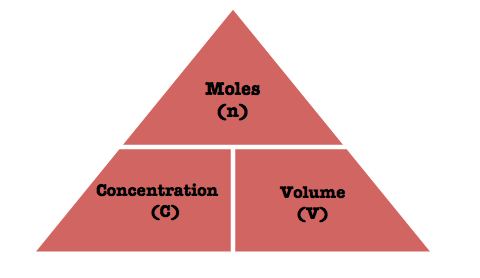
Concentration and Volume
Different units of measurement for this formula triangle is:
- Concentration – Molarity
- Volume – dm3 or litres
- Avogadro’s Law is that 1 mole of any gas occuplies the same volume under the same conditions and, at room temperature and pressure, 1 mole of gas is always 24 litres. Molar volume is the volume of 1 mole of gas
- Room Temperature = 298K or 1 atmosphere (atm)
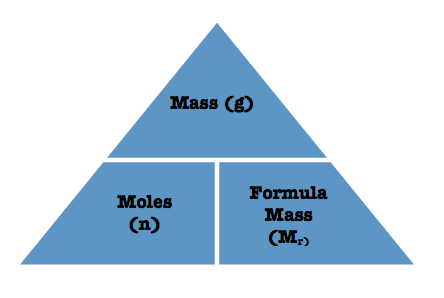
Mass and Formula Mass
Different units of measurement for this formula triangle is:
- Mass = grams , kilograms, etc
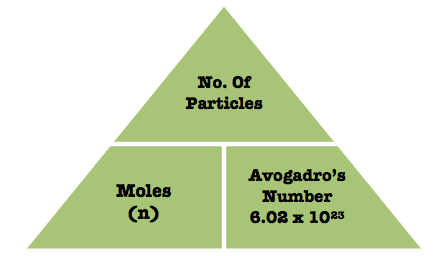
No. Of Particles
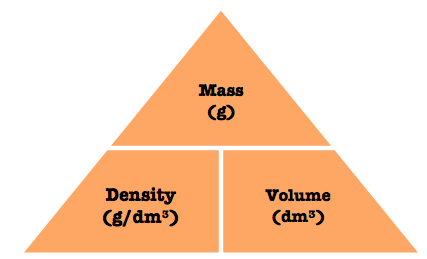
Density
This last triangle isn’t for mole conversion, but it’s helpful to know just in case.